The gut microbiota may be involved in ovarian dysfunction and insulin resistance in polycystic ovary syndrome
 Thomas AM & Segata N / BMC Biol 2019 (CC BY 4.0)
Thomas AM & Segata N / BMC Biol 2019 (CC BY 4.0)
Emerging evidence shows the involvement of our gut microbiome in conditions apparently unrelated to the gut ecosystem. And it seems that is the case with polycystic ovary syndrome (PCOS), which is a heterogeneous endocrine disorder that is often accompanied by altered insulin activity.
Recent studies have associated alterations in gut microbiome composition and barrier function with imbalances in the stool microbiome, gut permeability, and inflammatory status in women with PCOS.
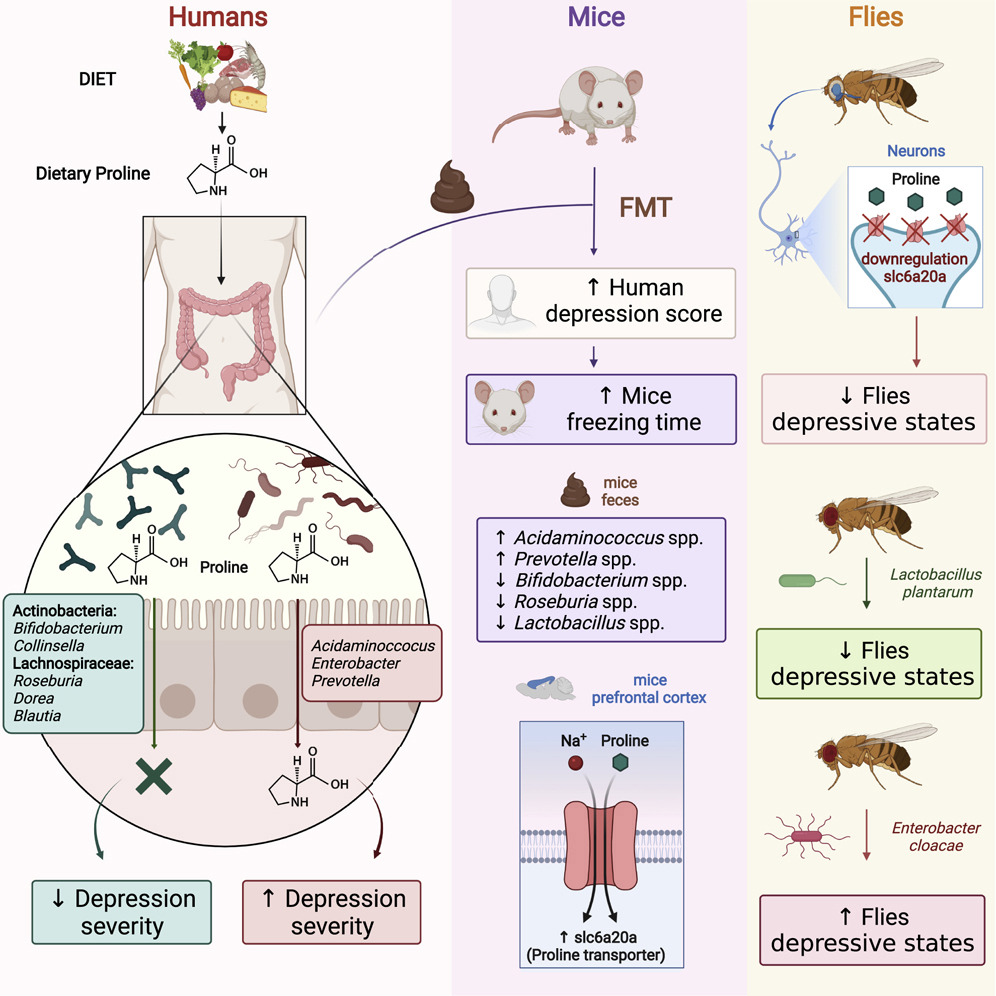
Researchers from Peking University (China), National Clinical Research Center for Obstetrics and Gynecology (China), National Institutes of Health (USA) and The Pennsylvania State University (USA) have shown that humanized mice receiving a fecal transplant from women with PCOS developed ovarian dysfunction, along with immune changes and insulin resistance.
The researchers first observed that women with PCOS showed a more homogenous gut microbiome and an enrichment in Bacteroides vulgatus, along with an increase in genes that encode bile salt hydrolases. Indeed, B. vulgatus was the gut bacterium with the highest discriminating power between women with PCOS and the control group.
At a functional level, it was shown that levels of glycodeoxycholic and tauroursodeoxycholic acid were reduced in the stool and serum of women with PCOS, which reflects an alteration of secondary bile acid biosynthesis.
Furthermore, the negative correlation of B. vulgatus with bile acid levels revealed that this gut bacterium may influence bile acid metabolic pathways.
In a second step, stool transplantation from women with PCOS or the administration of B. vulgatus by oral gavage induced insulin resistance, a disrupted estrous cycle and ovarian morphology, and changes in testosterone and luteinizing hormone levels in recipient mice. Furthermore, tauroursodeoxycholic and interleukin-22 messenger ribonucleic acid levels were markedly lower in the intestine. This was accompanied by decreased levels of a subset of immune cells in the small intestine lamina propria (called 3 innate lymphoid cells), involved in the production of IL-22.
These findings reveal that the gut microbiota may have a causal role in mediating disrupted insulin sensitivity and ovarian function in PCOS.
The administration of IL-22 and bile acid glycodeoxycholic acid improved insulin resistance, ovarian dysfunction and infertility in mice with PCOS. The authors also noted the contribution of bile acids in IL-22 expression, which was specifically mediated by GATA binding protein 3—required for the function of IL-22-producing immune cells—adipose tissue browning and the inhibition of inflammation in PCOS granulosa cells.
These data highlight that the decrease in glycodeoxycholic acid and subsequent decreased IL-22—also documented in women with PCOS when compared with healthy controls—may be involved in the metabolic and ovarian deterioration that characterizes PCOS.
In conclusion, this study provides the first evidence of the causal contribution of gut microbiota composition and disruption in secondary bile acid biosynthesis as new players in the pathogenesis of PCOS. As no effective treatment is yet available for this condition, these findings may pave the way for the development of new therapeutics that specifically target IL-22, glycodeoxycholic acid and the gut microbiota.
Reference:
Xinyu Qi, Chuyu Yun, Lulu Sun, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019. doi: 10.1038/s41591-019-0509-0.
News source: https://www.gutmicrobiotaforhealth.com